Your Carbon Dioxide Safety Resource Center
Safe Use of Carbon Dioxide in Cannabis Facilities, Food & Beverage Service, and Other Environments
Understanding Carbon Dioxide Hazards
Carbon dioxide (CO2) is used in a wide range of applications. It is unique because it is commercially used in several forms: as a gas for applications such as carbonation, purging, and pH control; as a liquid for applications including food processing and fire suppression; as a solid for applications such as expendable refrigeration and chilling; and as a supercritical fluid for applications including specialized extraction processes for substances such as caffeine and cannabis.
Dry ice is the solid form of carbon dioxide. It is used extensively as a cooling agent in a variety of applications such as food chilling and freezing, vaccine cooling, expendable refrigeration, blood and tissue sample preservation, metal heat treating processes, and many more. It is also sometimes used to create special effects such as fog for events or stage presentations. For more information about dry ice, see our online Dry Ice Safety Resource Center.
Anyone who handles carbon dioxide should be aware of its unique properties and potential hazards. Carbon dioxide is 1.5 times heavier than air and tends to accumulate in low or confined areas. It can displace oxygen and cause rapid suffocation as well as other serious physiological effects.
In addition, carbon dioxide can pose additional hazards such as exposure to extremely cold vapors or liquid or dry ice blockage of piping depending on its physical state.
The container label and safety data sheet (SDS) provide detailed hazard information and handling precautions. Always read and understand the label and the SDS before using any product and follow the instructions and safety precautions provided by your product supplier. These safety posters, provided by the Compressed Gas Association, provide basic safety information about the hazards associated with carbon dioxide. To learn more, see the section, “Product Information: Carbon Dioxide” at the bottom of this page.
Carbon Dioxide Safety Reminders
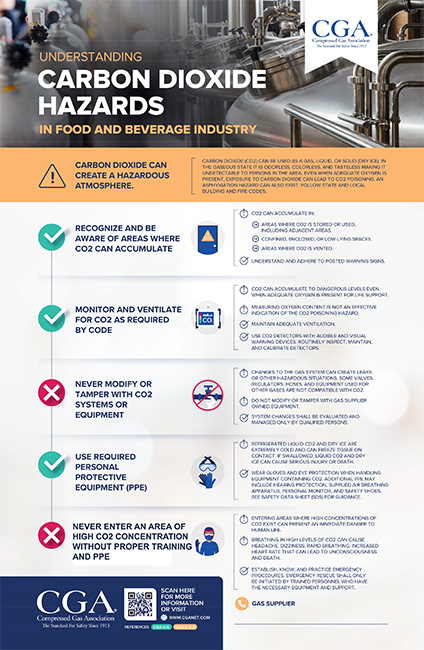
Carbon dioxide can create a hazardous atmosphere.
- Recognize and be aware of areas where carbon dioxide can accumulate.
- Monitor and ventilate for carbon dioxide as required by code.
- Never modify or tamper with carbon dioxide systems or equipment.
- Use required personal protective equipment (PPE) when working with carbon dioxide.
- Never enter an area of high carbon dioxide concentration without proper personal protective equipment (PPE).
Poster Downloads
CGA offers safety posters as educational resources to support the safe use of our industry’s products and equipment. It is important to note that these posters are not a substitute for reading and following codes and regulations, industry standards, and supplier instructions. Download your free carbon dioxide safety poster today!
NOTE – Use self-print files for printing at your home or office, and full bleed files for professional printing.
Additional Resources
CGA Publications
- CGA G-6, Carbon Dioxide
- CGA G-6.9, Dry Ice
- CGA G-6.1, Standard for Insulated Liquid Carbon Dioxide Systems at Consumer Sites
- CGA G-6.5, Standard for Small Stationary Insulated Carbon Dioxide Supply Systems
- CGA G-6.7, Safe Handling of Liquid Carbon Dioxide Containers that Have Lost Pressure
Free CGA Safety Materials
Product Information: Carbon Dioxide
Carbon dioxide (CO2) is a compound of carbon and oxygen. A gas at normal atmospheric pressures and temperatures, carbon dioxide is colorless, odorless, slightly acidic, and about 1.5 times heavier than air. It can exist simultaneously at its triple point as a solid (dry ice), liquid, and gas at a temperature of –69.9 °F (–56.6 °C) and a pressure of 60.4 psig (416 kPa). At temperatures and pressures below the triple point, carbon dioxide can be either a solid or a gas, depending upon temperature conditions. At temperatures and pressures above the triple point and below 87.9 °F (31.1 °C), carbon dioxide liquid and gas can exist in equilibrium in a closed container. The solubility of carbon dioxide in water varies with temperature and pressure.
Carbon dioxide does not support life and can be dangerous even when adequate oxygen is available.